FDA Approves Tirbanibulin for Actinic Keratosis on Expanded Area of Face or Scalp
Tirabanibulin (Klisyri; Almirall) has been licenced by the FDA to treat actinic keratosis in wider areas of the face or scalp up to 100 cm2.
The microtubule inhibitor ointment tirbanibulin has been licenced as a topical field treatment for actinic keratosis of the face or scalp for five days. It comes in a 350 mg package size. A news release states that the new approval raises the prior dose for surface area treatment from 25 cm2 to up to 100 cm2.
Karl Ziegelbauer, chief scientific officer at Almirall, stated in a news release that “the FDA’s approval of the use of Klisyri for actinic keratosis on an extended surface of the face or scalp is a significant step forward for both patients and treating dermatologists.” “Dermatists are searching for ways to treat the entire affected area to help prevent further lesion progression when patients experience [actinic keratosis] over larger surface areas.”
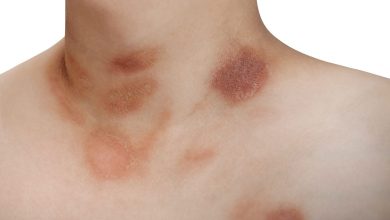
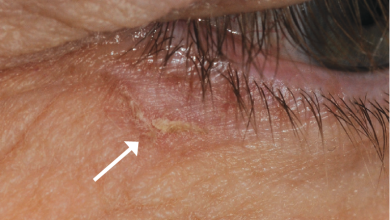
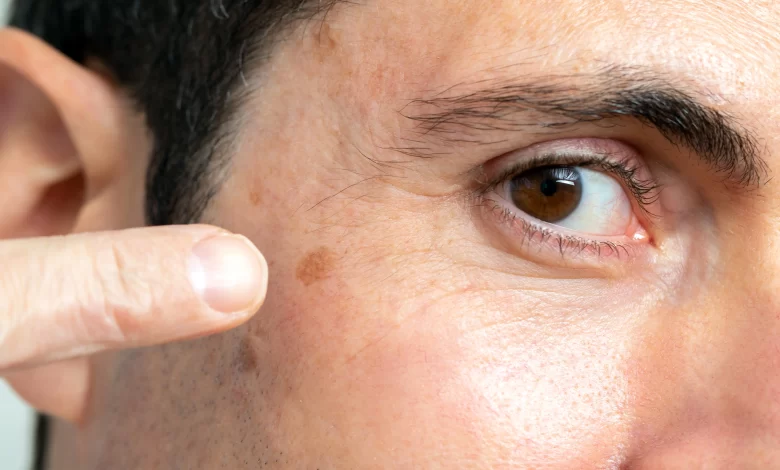
A rough, scaly area or bump on the skin brought on by UV exposure is known as actinic keratosis. Notably, actinic keratoses are frequently referred to as precancerous lesions since they have the potential to develop into squamous cell skin cancer. The face, ears, bald head, neck, backs of hands and forearms, lips, and other parts of the skin that are frequently exposed to the sun are the places where it typically manifests.
Actinic keratosis can be treated with cryotherapy, topical chemotherapy, laser surgery, or other lesion-destroying or removal techniques. Actinic keratoses are usually curable, however they might occasionally recur. Encouraging patients to undergo routine skin examinations even following treatment is crucial.
An additional phase 3, multicenter, open-label clinical safety trial including over 100 patients in the US forms the basis for the clearance. The main goals were to assess the safety and tolerability of administering tirbanibulin to adult patients with actinic keratosis’s face or balding scalp in a field measuring roughly 100 cm2. According to the study’s findings, the trial’s outcomes for local skin responses and treatment-related adverse events (AEs) were consistent with those of the initial pivotal studies.
Research on tirbanibulin’s effectiveness in a broader region revealed a percent decrease across the number of actinic keratosis lesions, which was comparable to the results of the initial pivotal studies.
Neil Bhatia, MD, the principal investigator for the larger treatment area pivotal study, stated in the news release that “clinicians can now treat up to 4 times the surface area, allowing increased flexibility to provide treatment of actinic keratoses and achieve effective results with a good safety and tolerability profile for more patients.”
According to a news release, tirbanibulin’s initial clearance in December 2020 was predicated on data from one of the biggest phase 3 clinical trial programmes ever carried out for a topical actinic keratosis treatment. A total of 702 participants were enrolled in the 2 studies at 62 US sites. Comparing patients treated with tirbanibulin to those treated with the vehicle, the former showed a considerably greater percentage of patients with actinic keratosis lesions completely cleared (44% vs 5% in study 1 and 54% vs 13% in study 2). Additionally, the secondary end target of partial (≥75) lesion removal was met.
The initial pivotal studies also showed a favourable safety profile; application site pruritis and discomfort occurred in 9% and 10% of patients treated with tirbanibulin, respectively, and were the most prevalent adverse events (AEs). AEs did not cause any patient to withdraw from the study.
References:
1. FDA approves Almirall’s Klisyri (tirbanibulin) for the treatment of actinic keratosis on expanded area of face or scalp up to 100 cm2. News release. Almirall. June 10, 2024. Accessed June 10, 2024. https://www.almirall.com/newsroom/news/fda-approves-almirall-s-klisyri-tirbanibulin-for-the-treatment-of-actinic-keratosis-on-expanded-area-of-face-or-scalp-up-to-100-cm2
2. Actinic Keratosis (A Precancerous Condition). Johns Hopkins Medicine. Accessed June 10, 2024. https://www.hopkinsmedicine.org/health/conditions-and-diseases/actinic-keratosis#:~:text=Actinic%20keratosis%20is%20a%20rough,into%20squamous%20cell%20skin%20cancer.
3. Almirall announces FDA approval of Klisyri (tirbanibulin), a new innovative topical treatment for actinic keratosis. News release. Almirall. December 15, 2020. Accessed June 10, 2024. https://www.almirall.com/newsroom/news/almirall-announces-fda-approval-of-klisyri%C2%AE-tirbanibulin-a-new-innovative-topical-treatment-for-actinic-keratosis